Due to the lack of transparency from manufacturers like GSK and Merck, there is concern about the safety of the HPV vaccines.
In a recent article, the HART Group reviews the evidence and highlights the known risks, acknowledged and unacknowledged uncertainties about the vaccine’s safety and the unknown risks of the vaccine from available data.
Let’s not lose touch…Your Government and Big Tech are actively trying to censor the information reported by The Exposé to serve their own needs. Subscribe to our emails now to make sure you receive the latest uncensored news in your inbox…
The Health Ethics Advocacy and Research Team (“HART Group”) has been publishing a series of articles on the “HPV vaccine strategy.” The following is Part 2, ‘HPV Vaccine Safety’. You can read Part 1, ‘HPV Vaccine Efficacy’, HERE and Part 3, ‘HPV Vaccines and Ethics’, HERE.
HPV Vaccine Safety
By HART Group
As with all medicines, the best evidence for safety will be from properly controlled randomised clinical trials with sufficient follow-up time. For vaccines in an otherwise healthy population, such trials need to be large and long and would not fit with the pharmaceutical business model. Regulators, therefore, become responsible for safety monitoring but do not put systems in place to ensure a fair control group to enable proper measurement. The consequences are that safety issues of a variety of types occur.
Lack of Transparency
GSK is the manufacturer of the other main HPV vaccine, Cervarix. GSK has been caught out in not being transparent with their safety data in the past. For example, Indian researchers noted that “Sudden Unexpected Deaths” in young children were reported in GSK’s periodic safety update reports (“PSURs”) for their Infanrix Hexa vaccine but the figures were changed between consecutive reports, leading to the disappearance of the safety signal. The researchers concluded, “There is a need to reappraise the reliance on due diligence by the EMA [European Medicines Agency].”
In one evidence review of HPV vaccines, it was noted that the majority of the trials gave the aluminium adjuvant to the control group rather than a saline placebo. The ethics of giving an aluminium adjuvant to the control group, with therefore potential for harm but no potential for benefit, has been called into question, quite apart from the potential for obscuring adverse events.
The Cochrane review found that deaths occurred more often among HPV vaccine recipients than in comparator groups. In women older than 25 years, the death rate was statistically higher in the vaccinated cohort (risk ratio 2.36, 95% CI 1.10–5.03), although no absolute counts for this subgroup were given. Overall, across all trials combined, there were 51 deaths in the HPV vaccine arms and 39 in the control arms. The Cochrane authors interpreted this imbalance as likely due to chance, noting no consistent pattern in causes of death or timing relative to vaccination. However, because the review included only randomised trials, they acknowledged they could not entirely exclude the possibility that vaccination contributed to the excess. The review also incorrectly refers to placebo controls, whereas in fact none of the papers they included used an inert placebo, with various adjuvants or other vaccines given to the control arm.
Critics of the review noted that many eligible trials were omitted by the Cochrane team, including a trial of the new Gardasil-9 vaccine, which was at that time the only published placebo-controlled trial. They also noted that deaths may sometimes be coded under unrelated categories – such as drowning or head injury – that could conceivably follow a syncopal episode, a recognised vaccine-associated event. Trials with up to 4 years follow-up only looked for serious adverse events in the first 14 days, the follow-up concentrating on efficacy rather than safety.
There has been particular criticism of Merck, which has apparently used a new and more potent adjuvant – amorphous aluminium hydroxyphosphate sulphate (“AAHS”) – in the Gardasil vaccine but they have refused to give details, claiming commercial sensitivity. Moreover, this was used as the “placebo” in one of their trials. Other aluminium adjuvants have been studied by independent scientists.
A useful framework for discussing these safety issues is Donald Rumsfeld’s “known knowns/unknowns” typology, adding in a fourth category of known but not acknowledged.
Known Knowns
Vaccine trials are well set up to detect acceptable known risks from vaccines, e.g. local reactions (pain, swelling, redness at injection site), mild systemic symptoms (fever, headache, fatigue). A risk of anaphylaxis has also been accepted as a “known known.”
Large population cohort studies (Scandinavia, US, UK, Australia, Japan) have consistently claimed no increase in autoimmune diseases, neurological syndromes or fertility and pregnancy issues at the population level.
The World Health Organisation (“WHO”), Centres for Disease Control and Prevention (“CDC”), EMA and Medicines and Healthcare products Regulatory Agency (“MHRA”) conclude HPV vaccines are “extremely safe,” with adverse event rates similar to other adolescent vaccines.
Known Unknowns
There are areas of acknowledged uncertainty. Current follow-up spans 15-17 years at most, so late-onset harms cannot yet be fully ruled out. There is little to no data on any particular subgroup with existing conditions to determine their risk. Finally, the vaccine is not licensed for use in pregnancy because data are still “not enough to be definitive.”
Unknown Knowns
In addition to the above, there is some data that is not officially acknowledged when seeking consent, which suggests there may be a level of harm beyond the known unknowns.
Disturbingly, the package insert leaflet notes that the product “may cause seizures and/or brain damage” as an adverse event. Acute disseminated encephalomyelitis, Guillain-Barré syndrome and immune thrombocytopenic purpura are listed as adverse events in the British National Formulary. Primary ovarian failure has also been reported with a 46-fold reporting ratio. This was particularly problematic as most of the trials involved only 2-4 weeks of follow-up. Even detecting a missed period would take longer than that, let alone diagnosing ovarian failure which could take several years to recognise. This is especially true when giving the vaccine to prepubertal girls.
Japan saw several legal cases launched in 2013 owing to the halting of clinical trials of the vaccine because of the level of harm. Harms and deaths from the vaccine have been reported in the drug safety reporting systems in many countries. Furthermore, the aluminium-containing adjuvants and emulsifiers in the HPV vaccinations (and many other vaccines) that are shown in the patient information leaflet and manufacturer’s warning list 26 adverse events.
Deaths
Worryingly, deaths are a genuine concern. The world-renowned experts on evidence-based medicine at Cochrane carried out a review of the trial evidence and showed that for women older than 25 years, the number of deaths from all causes in the vaccinated arm was more than double that in the placebo groups. For all ages, there was a suggestion of an increase overall but this was not statistically significant. Like the covid vaccine deaths, the reported deaths were not sudden cardiac deaths that were simply being misattributed, as there was often a clear syndrome of symptoms in the interim between vaccination and death. Furthermore, autopsy sampling showed inflammation in the vessels associated with the presence of vaccine-derived proteins.
Post-rollout safety monitoring is not a good way of measuring a fatality risk. To illustrate how insensitive a measure this is, we can model what it would look like. For example, using USA data from the period before and after the vaccine was introduced, the orange dots in the graph below show the actual deaths 2007-2011 against previous rates 2002-2006 HPV; vaccines were not recommended for boys in USA until 2011 and their deaths 2007-2011, while lower, match the profile by age of the deaths 2002-2006. The green dots show the same data but removing one hypothetical death each month of an 11 and one of a 12-year-old girl (the ages at which the HPV vaccine is given in the USA in girls); the difference in the overall deaths for the whole cohort (i.e. compared to the orange dots) is negligible. Even a 5% increase in deaths only equates to one extra death per month. It would take several deaths each month in this 11-12 age group for a change to be noticeable.
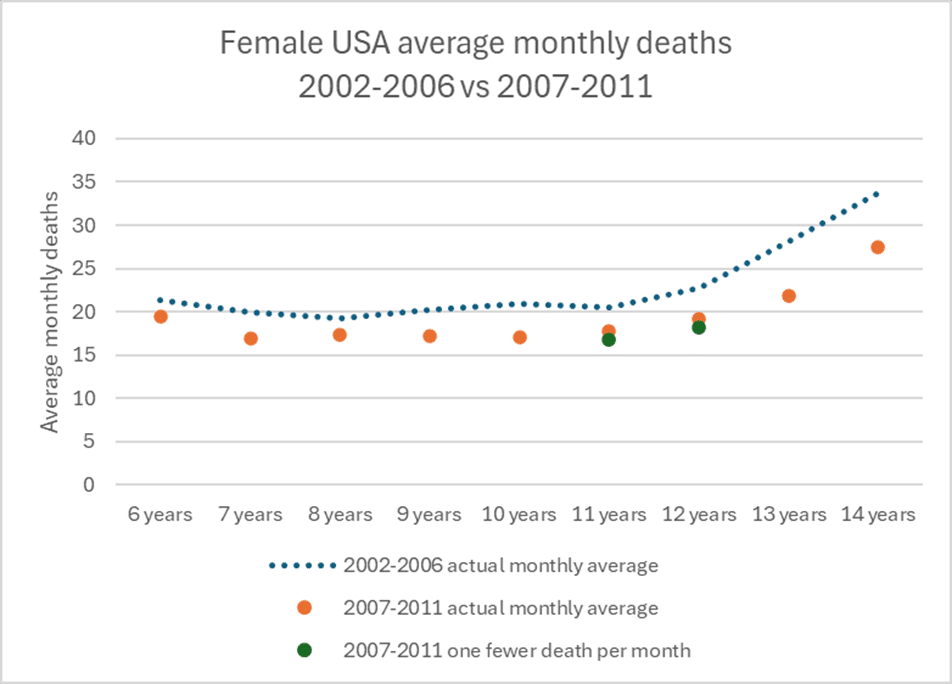
The bottom line is, clinical trials are the only fair measure, and when they show a signal, this should not be brushed aside.
Questions Around Autoimmune Disease and POTS
Others have written extensively on the harms of the HPV vaccine. Some case reports suggest autoimmune activation is temporally associated with vaccination. While causation isn’t supported by large studies, it remains a debated issue among some clinicians and patients. Clusters of Postural Orthostatic Tachycardia Syndrome (“POTS”) have also raised concerns, although large registry studies found no population-level signal.
DNA Contamination of Vaccines
A report in Italy, in what was labelled ‘Vaccinegate’, found a variety of contaminants including DNA of unidentified origins, in several different vaccines used across the world, including Gardasil 9. In an American study of 16 samples of Gardasil from around the world, DNA contamination was demonstrated in all 16, although the FDA dismiss this as of no clinical significance. Merck said they had been unable to replicate these findings. The same issues of the increase in the acceptable limit of DNA over time (raised in 1998 from 100pg to 10 ng per dose, i.e. 100x higher) and the failure to use the most sensitive testing methods to detect the DNA, apply as much here as to covid vaccines.
Unknown Unknowns
Finally, as always, we do not know what we do not know. When proton pump inhibitors, e.g. omeprazole and lansoprazole were widely used to treat peptic ulcers and reduce oesophageal cancer risk, there was an unanticipated increase in stomach cancer. Similarly, we do not understand any unexpected benefits from HPV to e.g. to immune responses and other risks. Given what happened with proton pump inhibitors, it would be naive to dismiss the possibility of unexpected harms out of hand.
As always with any drug, the population data needs translating into a number needed to treat and a number needed to harm. It is difficult to carry out such sums without extensive data. The number needed to treat remains unknown given that, if it works as hoped, the main benefit will be seen in saving the lives of older women. The number needed to harm remains unknown because of trials that did not have inert placebo controls and did not have extensive follow-up. The result is that decision-making is very hard. The public deserves far better experimental work to answer these fundamental questions.
Professor Diane Harper, who was the lead researcher in the development of the HPV vaccines in the USA, stated candidly in her 2010 review that physicians must ask, “Is there good evidence that this new vaccine is likely to make my patient live longer or better compared with the available alternatives?”
About the Author
HART is a group of highly qualified UK doctors, scientists, economists, psychologists and other academic experts. This group came together over shared concerns about policy and guidance recommendations relating to the covid pandemic.
The group continue to be concerned about the lack of open scientific debate in corporate media and the worrying trend of censorship and harassment of those who question the narrative. “Science without question is dogma,” HART says.
The Expose Urgently Needs Your Help…
Can you please help to keep the lights on with The Expose’s honest, reliable, powerful and truthful journalism?
Your Government & Big Tech organisations
try to silence & shut down The Expose.
So we need your help to ensure
we can continue to bring you the
facts the mainstream refuses to.
The government does not fund us
to publish lies and propaganda on their
behalf like the Mainstream Media.
Instead, we rely solely on your support. So
please support us in our efforts to bring
you honest, reliable, investigative journalism
today. It’s secure, quick and easy.
Please choose your preferred method below to show your support.
Categories: Breaking News, World News

The vaccine manufacturers must be held liable and accountable for deaths and injuries their products cause. Currently, the vaccine manufacturers have full immunity and can do what they want. Before 1986, the vaccine manufacturers could be sued and held accountable for harms their products caused. Since 1986, we have seen an explosion in all kinds of vaccines for everything under the sun. The human immune system, doesn’t stand a chance of developing if it is constantly attacked with all these aluminium and other highly toxic chemicals, DNA and other harmful substances.
There needs to be laws in place and these vaccines should be halted, yes all of them, until the laws protect the people, rather than the vaccine manufacturers.
If I am not mistaken, there is a Gardisil trial going on right now in the U.S. with Peter Goetzsche leading the charge (that this “vaccine” causes a great deal of harm). Are you familiar with his books and his personal history (i.e., that he was one of the founders of the Cochrane Collection and they ousted him later for his honesty and good scholarship)?